Science Source
Ocean Acidification: Present Conditions and Future Changes in a High-CO
- States the uptake of anthropogenic CO2 by the global ocean induces fundamental changes in seawater chemistry that could have dramatic impacts on biological ecosystems in the upper ocean
- States that estimates based on the Intergovernmental Panel on Climate Change (IPCC) business-as-usual emission scenarios suggest that atmospheric CO2 levels could approach 800 ppm near the end of the century
- States that corresponding biogeochemical models for the ocean indicate that surface water pH will drop from a pre-industrial value of about 8.2 to about 7.8 in the IPCC A2 scenario by the end of this century, increasing the ocean’s acidity by about 150% relative to the beginning of the industrial era
- Holds that in contemporary ocean water, elevated CO2 will also cause substantial reductions in surface water carbonate ion concentrations, in terms of either absolute changes or fractional changes relative to pre-industrial levels
- Holds that for most open-ocean surface waters, aragonite undersaturation occurs when carbonate ion concentrations drop below approximately 66 µmol kg-1
- Model projections indicate that aragonite undersaturation will start to occur by about 2020 in the Arctic Ocean and 2050 in the Southern Ocean
- Finds that by 2050, all of the Arctic will be undersaturated with respect to aragonite, and by 2095, all of the Southern Ocean and parts of the North Pacific will be undersaturated
- Finds that for calcite, undersaturation occurs when carbonate ion concentration drops below 42 µmol kg-1
- Finds that by 2095, most of the Arctic and some parts of the Bering and Chukchi seas will be undersaturated with respect to calcite
- Finds, however, in most of the other ocean basins, the surface waters will still be saturated with respect to calcite, but at a level greatly reduced from the present
Related Content
Headline
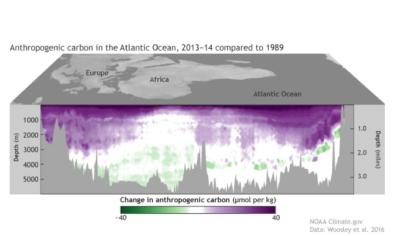
Jun 6, 2018 | NOAA Climate.gov
Cruises cut a slice through the Atlantic's carbon pie
Science Source
| Nature Climate Change
Detecting regional anthropogenic trends in ocean acidification against natural variability
T. Friedrich, A. Timmermann, A. Abe-Ouchi et al
Science Source
| Biogeosciences
Using present-day observations to detect when anthropogenic change forces surface ocean carbonate chemistry outside preindustrial bounds
Sutton, Adrienne J., Sabine et al
Science Source
| Estuaries and Coasts
Is Ocean Acidification an Open-Ocean Syndrome? Understanding Anthropogenic Impacts on Seawater pH
Carlos M. Duarte, Iris E. Hendriks, Tommy S. Moore et al


